Research
Autophagy Aarhus brings together the research teams of Fulvio Reggiori and Muriel Mari, two groups with a long-standing history of synergistic collaboration and scientific innovation. Together, we investigate fundamental questions in autophagy and related cellular pathways, using cutting-edge approaches to understand how cells maintain their health and respond to stress. Our research spans a broad range of cell biology and thrives on strong partnerships with national and international collaborators.
We are an active, interdisciplinary community committed to discovery, training, and scientific excellence, and we are always excited to welcome new talent to join us on this journey.
The mechanism of autophagy
We use yeast Saccharomyces cerevisiae as the model system to uncover the molecular function of the autophagy-related proteins and study the membrane rearrangements that lead to the de novo formation of autophagosomes.
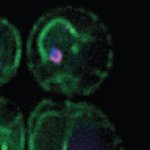
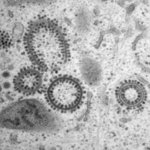
The interaction between viruses and autophagy/autophagy-related
We study autophagy-virus interactions. In this framework, we were among the first to discover that autophagy-related proteins also operate outside the context of autophagy. This novel research area has important implications, as ATG proteins with multiple functions might be regulatory hubs. We also exploit viral infections to identify novel unconventional functions of autophagy-related proteins.
Autophagy in preventing neurodegeneration
We investigate the mechanism underlying aggrephagy, the selective degradation of aggregates by autophagy, which could be relevant in the development of strategies preventing neurodegeneration. Here, we benefit from a new system that allows monitoring aggrephagy in real time.