Insights
Single-Cell Secretion Assays
Many immune functions rely on secreted proteins—cytokines, chemokines, enzymes, antibodies—so we developed specialized assays to quantify these factors at the single-cell level using droplet microfluidics.
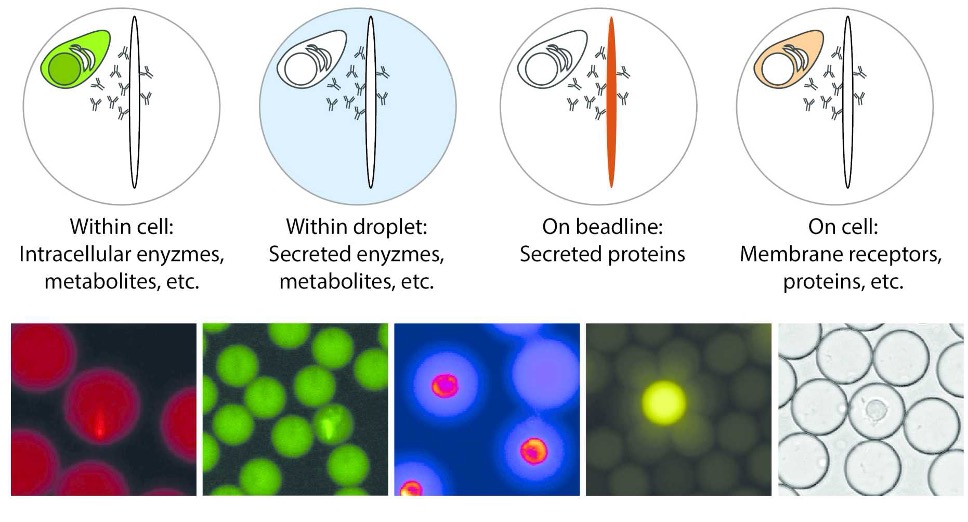
Our method uses an in-droplet sandwich immunoassay with paramagnetic nanoparticles pre-coated with capture antibodies. Each droplet contains a single cell, a detection antibody, and hundreds to thousands of magnetic beads.
Beadline-Based Functional Readout
When a cell secretes the target protein, it binds to the beadline (an elongated structure formed by magnetic alignment), attracting the fluorescent detection antibody.
We quantify secretion by measuring fluorescence relocation onto the beadline over time, providing real-time data on secretion rates and functional activity for each cell. This approach also allows automated detection of secreting vs. non-secreting cells, enabling population-level analysis.
Multiplexing and Expansion
Our platform supports multiplexed detection of up to 5–6 different functions in parallel, including secretion of cytokines (e.g., TNF-α, IFN-γ, IL-2, IL-6), antibody isotypes, membrane protein shedding, metabolic fluxes, and more.
We continue to expand this technology to track even more complex immune behaviors at single-cell resolution.