Novel Insights into Neurological Diseases through Functional Single-Cell Analysis in the Cerebrospinal Fluid
Project Overview
- Focus: Investigating immune responses within the central nervous system via cerebrospinal fluid (CSF)
- Why It Matters: CSF offers a unique and clinically valuable window into brain immunity and disease processes.
Current Challenges
- Traditional CSF analysis relies on bulk measurements (cytokine levels, cell counts)
- These methods often miss cellular complexity and functional heterogeneity
- Single-cell function remains difficult to access due to low cell counts in CSF
Our Approach
- Innovation: Functional single-cell analysis using droplet-based microfluidics
- Goal: Quantify cytokine and antibody secretion at the single-cell level
Expected Outcomes
- High-resolution mapping of immune dynamics in CSF
- Identification of novel immune cell phenotypes
- Improved disease classification
- Deeper insight into neuroinflammatory mechanisms
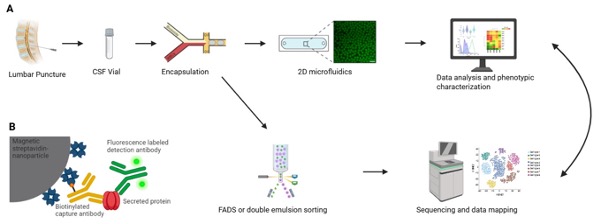
A. Experimental workflow of phenotypic/genotypic measurements of CSF samples
CSF vials from patients are obtained from a lumbar puncture. A heterogenous cell population is isolated from the vial and encapsulated together with assay reagents into 60 pL water-in-oil emulsions (droplets). After encapsulation, the droplets are subjected to customized 2D phenotype assays for the dynamic characterization of cellular activity, using sandwich immunoassays and time-resolved fluorescence measurements, followed by a customized data analysis pipeline, allowing in-depth characterization of immune activities. Alternatively, one part of the droplets can be used to sort according to the phenotype detected by the bioassay with either fluorescence activated droplet sorting (FADS) or via re-emulsification and commercial cell sorters. The enriched cells are then subjected to commercial sequencing approaches. Thus, this workflow allows for the detailed comparison of pheno- and genotypes in the measured samples.
B. Principle of the deployed bioassay
Nanoparticles are functionalized with specific capture antibodies, leading to the accumulation of the secreted protein onto the nanoparticles. Fluorescently labeled detection antibody subsequently relocates onto the nanoparticles as well, leading to a measurable and quantifiable fluorescence accumulation. Figure was created using Biorender.