Endothelial cell heterogeneity
The vascular system consists of not only distinct vascular beds, but also heterogeneous phenotypes, presumably to meet the tissues’ various physiological needs. For instance, the blood-brain barrier comprises tightly linked endothelial cells (ECs) to restrict paracellular diffusion, while permeable fenestrations in liver ECs facilitate the exchange of solutes. The available Endothelial Cell Atlas provides a transcriptional map that lets us explore EC heterogeneity in different organs. In our group we focus on scrutinizing this topic by looking at EC heterogeneity in different tissues (e.g. different depots of an adipose tissue); In doing so we use state-of-the-art approaches (e.g. single-cell RNA sequencing or spatially-resolved transcriptomics).
Metabolic adaptation during new vessels formation
Our laboratory investigates how the blood vessel growth (angiogenesis) is regulated by its own metabolism. We study how ECs ‘sense’ their (metabolic) microenvironment and how they use this information to build vessels of tissue-specific size and function. We aim to understand how changes in endothelial bioenergetics impact vascular growth and homeostasis in health and disease. To this end, we apply standard molecular biology techniques (e.g. CRISPR/Cas9 gene targeting /silencing/overexpression) as well as high-throughput multi-omics approaches (metabolomics, proteomics and bulk scRNAseq).
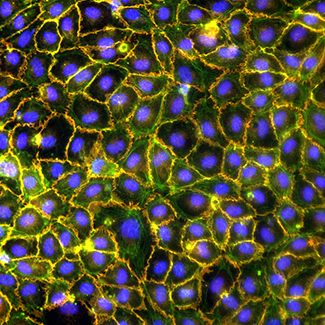
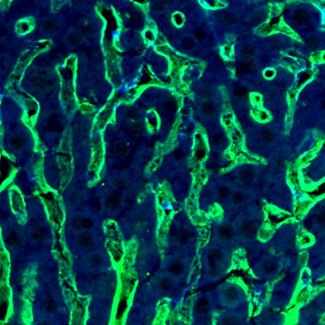
Endothelial cell biology during obesity and diabetic complications
Impaired vascular remodelling during the expansion of adipose tissue, promotes hypoxia and inflammation. These features lead to increased occurrences of obesity-related tissue malfunctions, including cardiovascular flaws. Therefore, dysfunctional blood vessels have become clinically recognized as a life-threatening incidence for humans. Given the emerging importance of understanding the biology of endothelium, we focus on characterization of ECs heterogeneity in adipose tissue. Our research will provide us with the answers to key questions, such as: What kind of impact the microenvironment represented by the obese state has on the gene expression in ECs. We collaborate on this with Steno Diabetes Centre Aarhus.
Obesity and cancer
There is a correlation between obesity and cancer. Epidemiological studies reveal a significant impact of obesity on increased cancer risk and worsened treatment prognosis. Moreover, obesity hinders both tumor-angiogenesis and antiangiogenic treatment in these patients. Understanding the molecular signaling in the obese setting might lead to a more targeted treatment of affected patients. In this project we aim to understand how obesity influences tumor-angiogenesis and the resistance to antiangiogenic treatment in cancer patients.