Research Interest
The Laboratory of Renal Cell Remodeling and Regeneration was established at the Department of Biomedicine in October 2019, and focuses on the role of renal cell remodeling in kidney disease and regeneration. We are approaching central questions of how renal cells cope with acute and chronic kidney injury, including renal cell turnover, fibrotic remodeling and cell death, using a unique optical approach. Thus, we use intravital multiphoton microscopy (MPM) and spinning disc confocal microscopy of the kidney in anesthetized mice to simultaneously access renal function and morphology at a subcellular resolution. Through implantation of an abdominal imaging window chamber, we further advance this approach to enable non-invasive serial imaging of the same renal cells over several days and weeks, allowing us to detect cell migration and proliferation based on paired observations. Furthermore, we combine advanced imaging techniques with a variety of other state-of-the-art techniques, such as complex transgenic mouse lines and in vivo kidney disease models, optical tissue clearing techniques; fluorescent activated cell sorting (FACS) and advanced sequencing techniques.
Endothelial cell plasticity in diabetic nephropathy
Diabetic nephropathy (DN) is a major complication in diabetes mellitus, the primary cause of end-stage renal failure and involves the deposition of extracellular matrix (ECM) in the tubule-interstitium, driving the progressing loss of renal function. ECM-accumulation in DN substantially derives from remodeling endothelial cells (EC), which undergo endothelial-to-mesenchymal-transition (EndoMT). It is therefore of utmost interest to better understand endothelial cell remodeling and signaling pathways in diabetic and control conditions. However, due to both, the lack of specific cellular biomarkers to distinguish early signs of EC remodeling and the lack of techniques, which allow for the dynamic tracking of ECs in vivo, our understanding of EC plasticity, is limited. Thus, the use of serial intravital MPM is instrumental as it allows to visualize these cells and their actions over time. We strive to better understand the underlying mechanisms involved in EC plasticity and how these can be manipulated using new and existing pharmacological tools.
Mechanisms of AKI-to-CKD transition
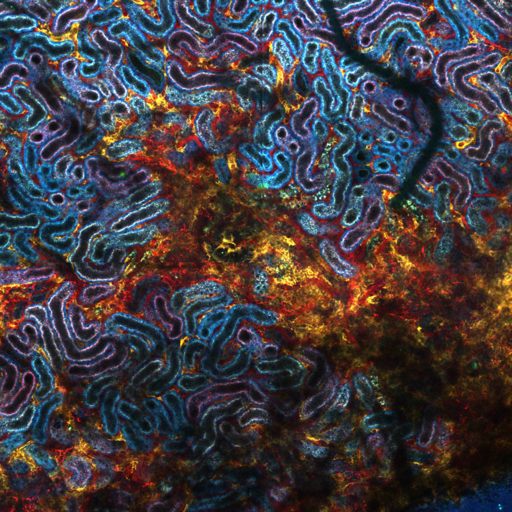
Even though the kidney is considered a rather stable organ with a limited cellular turnover rate, we know that it holds a remarkable regenerative capacity to recover from acute kidney injury (AKI). However, recovering AKI patients are at a higher risk of developing chronic kidney disease (CKD) and end-stage-renal disease. The underlying mechanisms of this AKI-to-CKD transition are only partially understood, but are suggested to involve incomplete repair mechanisms in the kidney. We seek to further understand endogenous kidney repair mechanisms, to identify and understand deregulatory pathways and to target them pharmacologically for improved renal functional recovery and prognosis.