Research
Project
Mapping niche dynamics at single-cell resolution to boost the regenerative capacity of skeletal muscle
Regenerative medicine aims to understand the cellular and molecular processes underlying effective tissue repair with the long-term goal to restore structure and function of damaged tissues and organs. Our body is able to repair almost any tissue and this capacity declines with aging. During aging, the ability of skeletal muscle to regenerate after acute injury declines and fibrosis takes over, leading to the replacement of damaged tissue with scar tissue, which holds the tissue together but does not restore function.
Effective regeneration, in skeletal muscle as well as in many other tissues, depends on the coordinated and timely interaction of stromal cells, immune cells and stem cells. This balanced interaction mediates the rapid transition from an inflammatory phase, critical for clearance of dying cells and debris, to a regenerative phase, during which muscle stem cells can expand and differentiate to repair damaged tissue. When this balance is disrupted fibrosis takes over, creating a barrier to effective muscle regeneration. Therefore, understanding how stromal cells, muscle stem cells and resident versus infiltrating immune cells interact to orchestrate effective muscle repair is key to develop therapeutic interventions for traumatic acute injury, aging and muscle diseases. A major barrier to address this question, has been the lack of single-cell technologies to simultaneously study the relationships between all the cells involved in a dynamic regeneration system, such as muscle injury.
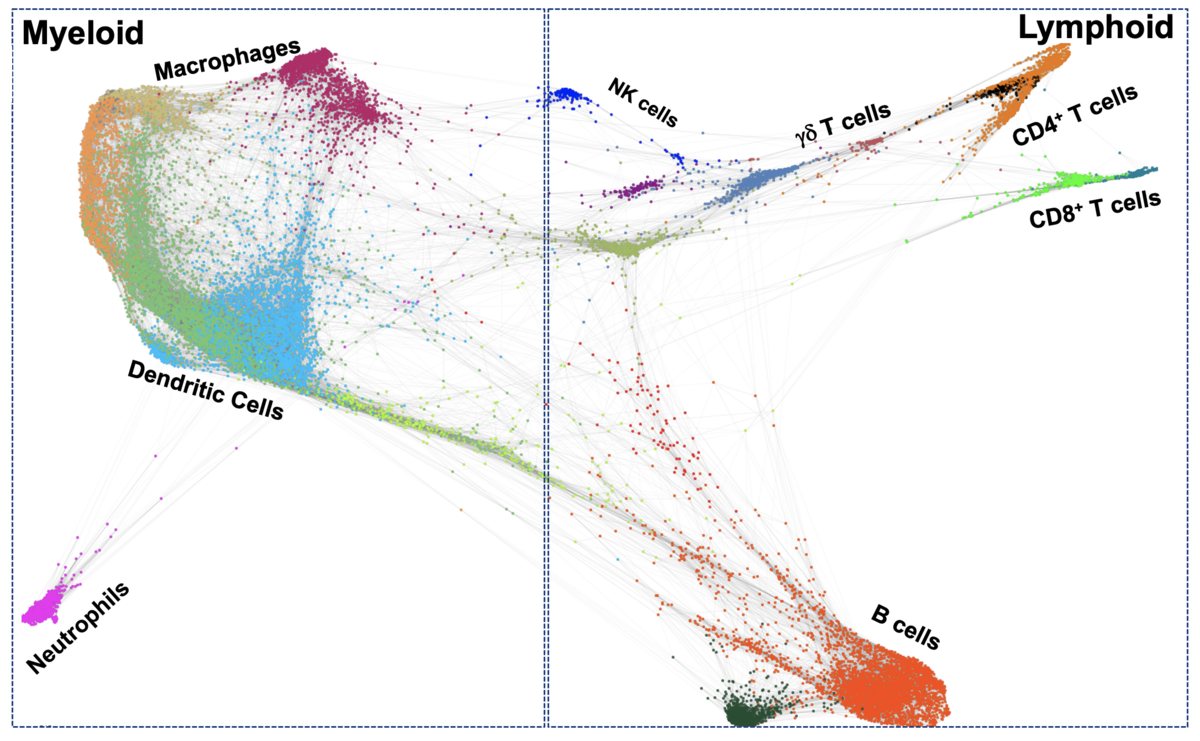
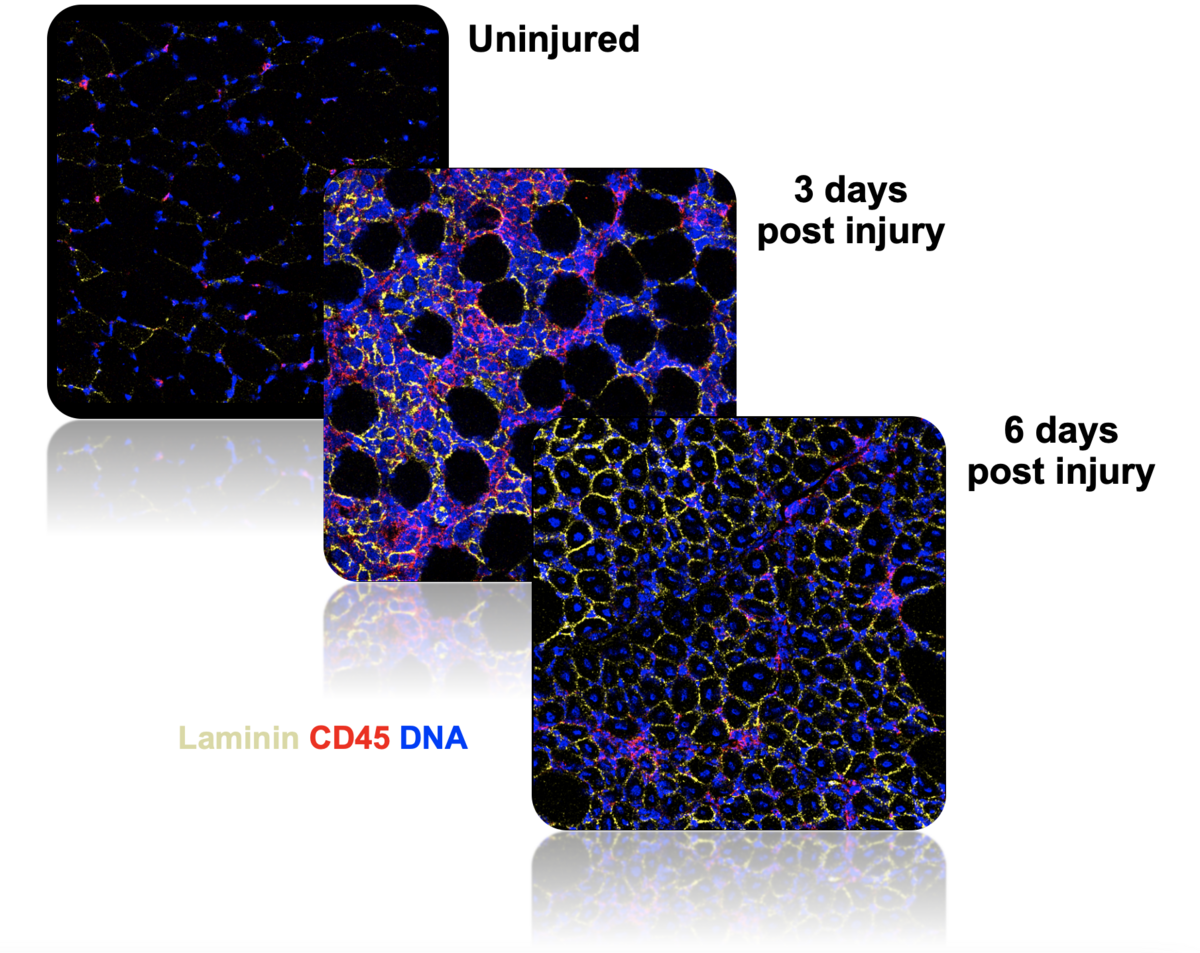
Our lab employs novel single-cell technologies, such as single-cell mass cytometry (CyTOF) and Imaging Mass Cytometry (IMC), to study the relationships between muscle stem cells and immune cells in aged and dystrophic skeletal muscle. Specifically, our research focuses on understanding how unique interactions between immune cells and muscle stem cells orchestrates effective muscle regeneration and on identifying the cellular interactions and signaling pathways that are impaired during ineffective muscle regeneration, in aging and muscular dystrophies. This research will help us develop strategies to modulate the immune system to boost muscle tissue repair.